Companion: monitor real-time anti-cancer drug adverse events using openFDA API
Click here to test the app!
openFDA provides a rich gateway for a variety of data sources that are related to public health.
Through an API, openFDA enables developers to fetch data of interest using API queries. Data is updated constantly,
therefore it gives you an access to near real-time information, such as drug and medical device adverse events.
Companion diagnostics are specialized medical devices that are essential for the
safe and effective use of a therapeutic product. They are typically used to stratify a potential
patient population (for instance by detecting biomarker, such as a cancer related gene mutation or
elevated levels of a protein)in order to identify the intended use population that is more
likely to benefit from the respective drug or biologic. Companion diagnostics are some of the
first examples of the concept called 'personalized (or precision) medicine'.
Since these devices are essential for the safe and effective use of these drugs,
I developed this web-based application to monitor adverse events that are associated with anti-cancer drugs and their
associated companion diagnostics.
Here is the architecture of this analytics tool to give you an idea how it works:
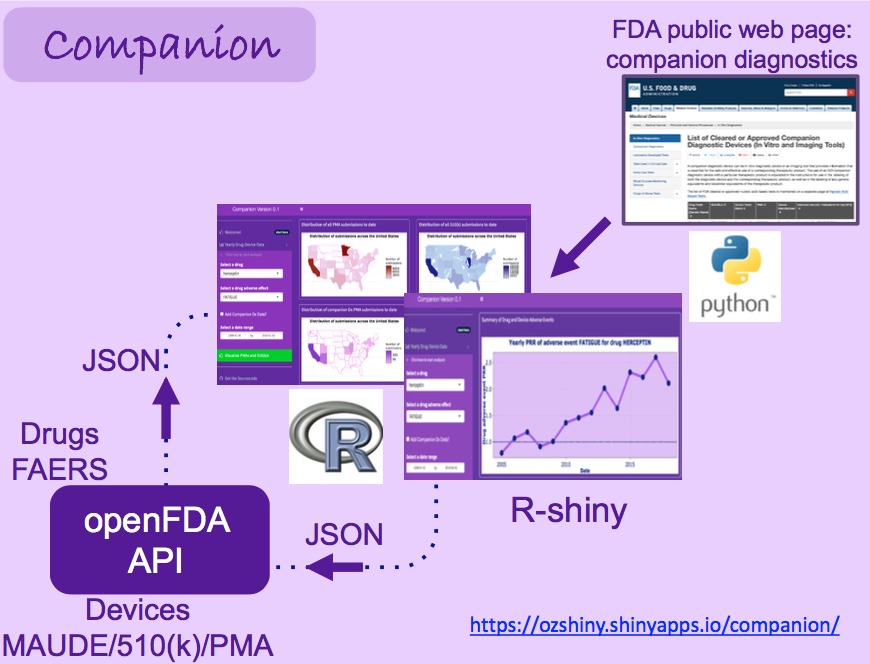
Whenever an anti-cancer drug is selected, the app will determine the companion diagnostic(s)
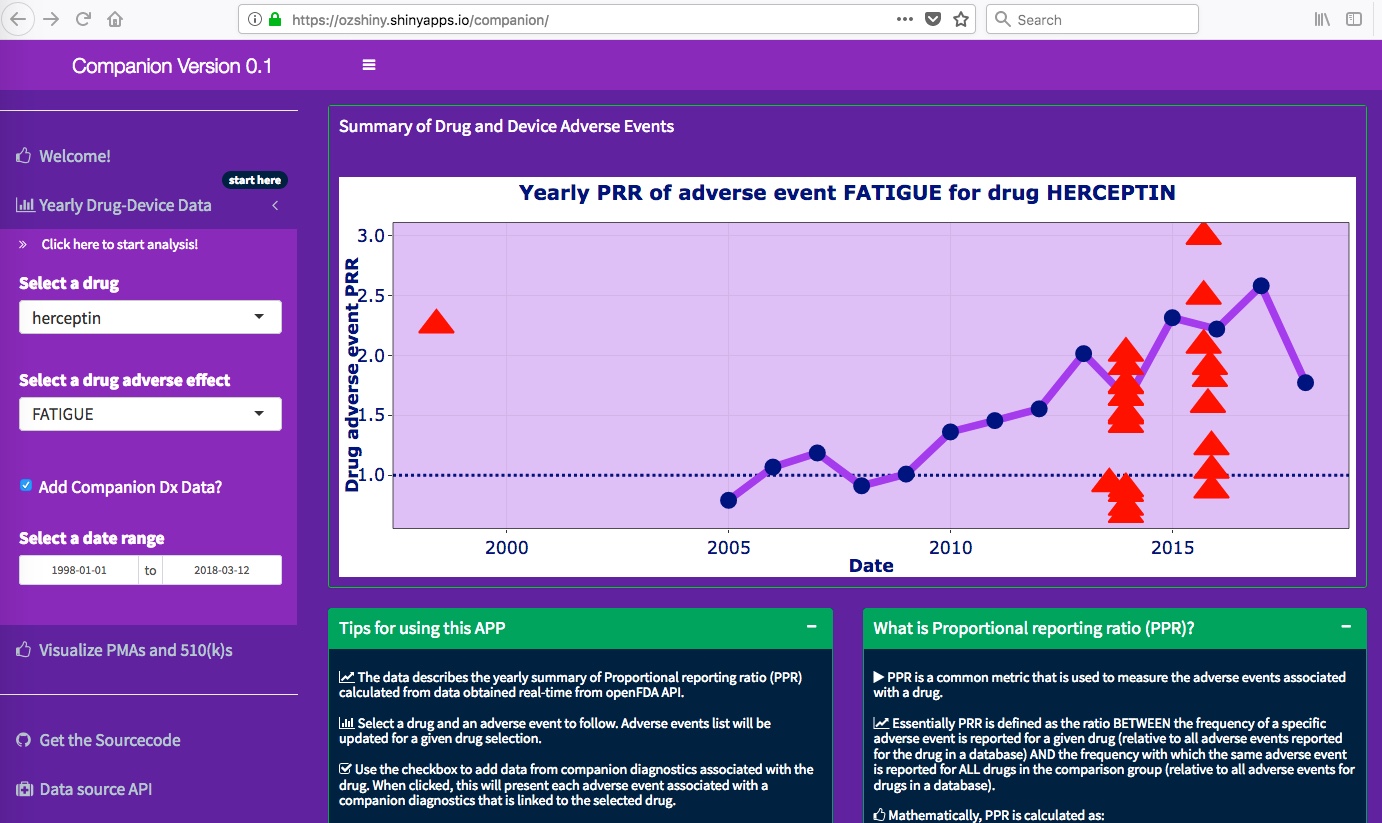
linked to this particular drug. The app will plot yearly PRR (Proportional Reporting Ratio) of a particular
adverse event associated with this drug. The adverse events associated with the respective companion
diagnostic device are overlaid as red-triangles. User can learn more about these events by hovering the
mouse on particular data points. The application enables monitoring pf PRR associated with different types of adverse events
reported for a particular drug to date, with the option of defining a particular time-window using a
calendar. Each time a specific drug/adverse event/time-window is specified, the app will make a
query to openFDA API and retrieve the most up-to-date adverse event information. This enables
real-time monitoring of adverse events.
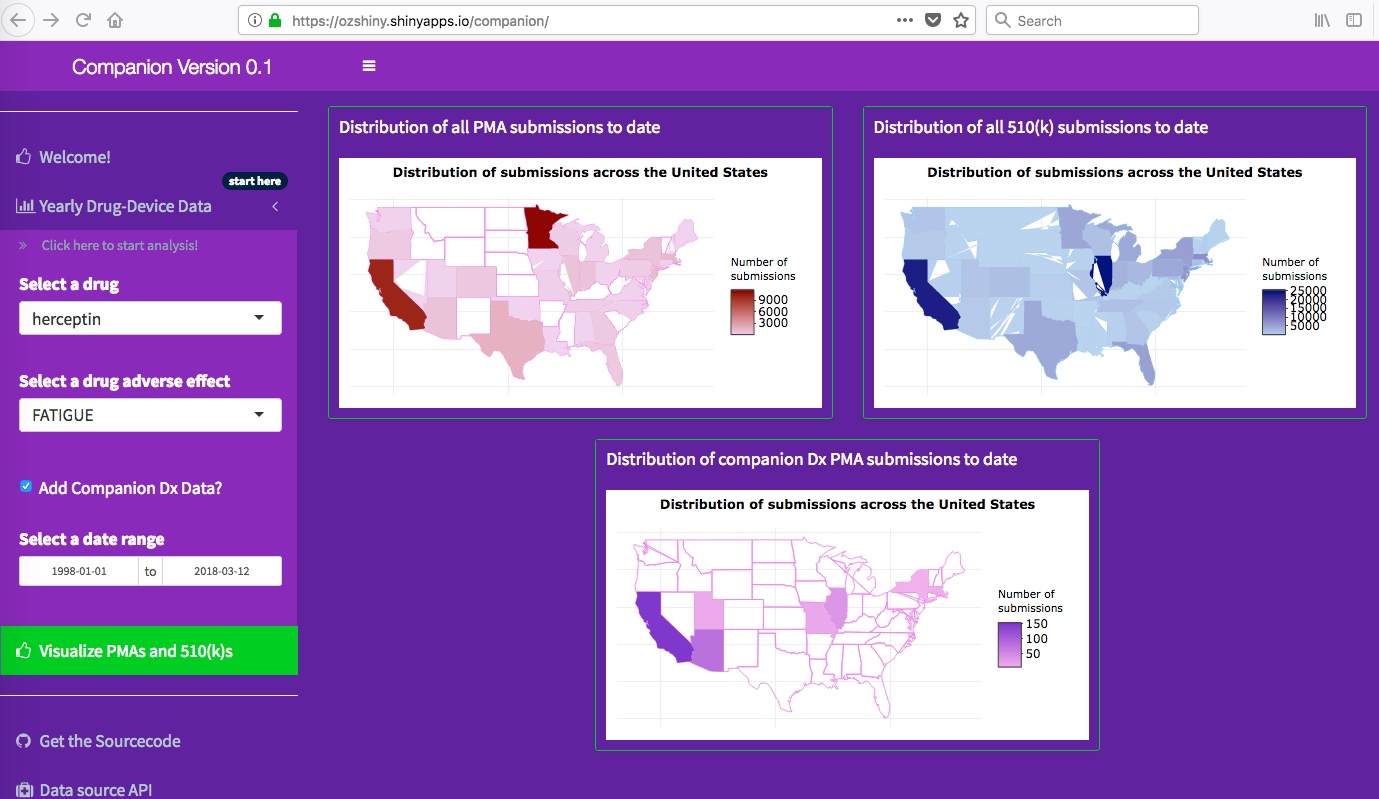
As an added feature, the app enables monitoring of PMAs or 510(k) device submissions
that are submitted from different geographical regions within the United States.
I hope you enjoyed learning about this tool. Feel free to check out the code I used to develop this app from
my GitHub page. It can also give you an introduction on how to fetch data from APIs and use JSON data to develop
dynamic applications in a R-shiny framework.


